Anthropic has launched Claude for Healthcare alongside significantly expanded life sciences features, bringing AI assistance to medical providers, payers, researchers, and patients through HIPAA-compliant infrastructure. The release marks a major expansion beyond the company's October announcement of Claude for Life Sciences, now covering the full spectrum from preclinical research to clinical trial management and regulatory operations.
Healthcare Provider and Payer Tools
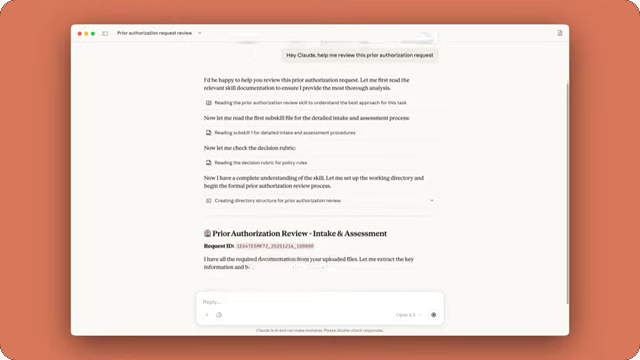
Claude for Healthcare introduces connectors to three healthcare databases: the Centers for Medicare & Medicaid Services (CMS) Coverage Database, the International Classification of Diseases, 10th Revision (ICD-10), and the National Provider Identifier Registry for credentialing and claims validation. Additionally, Anthropic has launched two new Agent Skills: FHIR development for improved healthcare system interoperability and customizable prior authorization review templates.
These integrations and skills enable healthcare organizations to streamline prior authorization reviews—a process that typically takes hours and delays patient care—by automatically cross-referencing coverage requirements, clinical guidelines, and patient records. The system can also support claims appeals by assembling necessary documentation from multiple fragmented sources, coordinate patient care by triaging portal messages and referrals, and help healthcare startups build new products, such as ambient clinical scribing tools.
Personal Health Data Integration
For individual users, Claude Pro and Max subscribers can now connect their personal health data through HealthEx, Function, Apple Health, and Android Health Connect integrations, available in beta. The system can summarize medical histories, explain test results in plain language, detect patterns across fitness metrics, and help prepare questions for doctor appointments. Anthropic emphasizes that these integrations are "private by design"—users control exactly what information they share, must explicitly opt-in, and health data is never used for model training.
Expanded Life Sciences Capabilities
In life sciences, new connectors extend Claude's reach beyond preclinical research to clinical trial operations and regulatory work. Integrations with Medidata enable tracking of trial enrollment and site performance, while access to ClinicalTrials.gov supports patient recruitment and protocol design. Additional connectors to bioRxiv, medRxiv, Open Targets, ChEMBL, and Owkin's Pathology Explorer provide researchers with comprehensive access to scientific literature, drug targets, bioactive compounds, and tissue analysis.
In parallel, new Agent Skills launched to support the life sciences include a sample skill for clinical trial protocol drafting, as well as skills converting instrument data to Allotrope and bundled skills for scVI-tools and Nextflow deployment.
Performance and Partnerships
Claude Opus 4.5 demonstrates substantial improvements on medical and scientific task simulations, with major pharmaceutical companies reporting transformative results. Novo Nordisk noted the work "set a new standard" for pharma development automation, while Sanofi reported efficiency gains across its entire value chain.
The expanded capabilities are available across Claude Pro, Max, Teams, and Enterprise plans, with deployment options spanning AWS, Google Cloud, and Microsoft Azure.






Comments